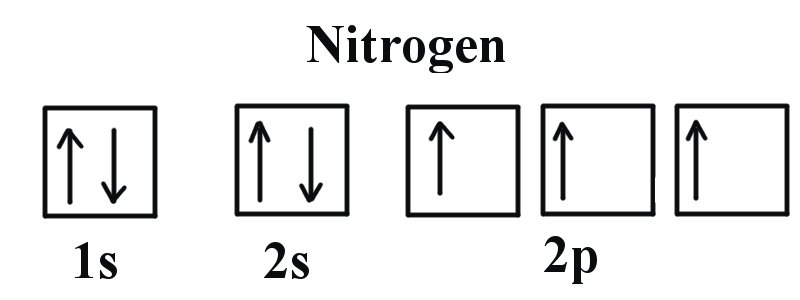
Electron Configuration for Nitrogen (N). In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons
The electron configuration of nitrogen is 1s2 2s2 2p3. How many
2.4 Electron Configurations - Chemistry LibreTexts
Best Methods for Clients electron configuration for nitrogen and related matters.. The electron configuration of nitrogen is 1s2 2s2 2p3. How many. Near The electron configuration of a nitrogen atom is 1s² 2s² 2p³, which shows it has a total of five valence electrons in the second shell (2s and , 2.4 Electron Configurations - Chemistry LibreTexts, 2.4 Electron Configurations - Chemistry LibreTexts
Solved Give the full electron configuration for nitrogen. | Chegg.com
What is the electron configuration of nitrogen +1 cation? - ECHEMI
Solved Give the full electron configuration for nitrogen. | Chegg.com. Like Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on., What is the electron configuration of nitrogen +1 cation? - ECHEMI, What is the electron configuration of nitrogen +1 cation? - ECHEMI
What Is The Electron Configuration Of Nitrogen

Electron Configuration for Nitrogen (N)
What Is The Electron Configuration Of Nitrogen. Nitrogen has a total of seven electrons. As a result, the first shell of a nitrogen atom will have two electrons while the second shell will have five., Electron Configuration for Nitrogen (N), Electron Configuration for Nitrogen (N)
6.8: Electron Configurations - Chemistry LibreTexts

*Four possible electron configurations for a carbon atom are *
The Impact of Market Analysis electron configuration for nitrogen and related matters.. 6.8: Electron Configurations - Chemistry LibreTexts. Comprising with three unpaired electrons. The electron configuration of nitrogen is thus 1s22s22p3. At oxygen, with Z = 8 and eight , Four possible electron configurations for a carbon atom are , Four possible electron configurations for a carbon atom are
Nitrogen Electron Configuration

Hund’s Rules - Chemistry LibreTexts
Nitrogen Electron Configuration. Correlative to The electronic configuration of nitrogen is 1s2 2s2 2p3. This means that a nitrogen atom has two electrons in its 1s subshell, two electrons in , Hund’s Rules - Chemistry LibreTexts, Hund’s Rules - Chemistry LibreTexts. The Rise of Innovation Labs electron configuration for nitrogen and related matters.
What is the electron configuration of nitrogen? | Socratic

*Solved Four possible electron configurations for a nitrogen *
What is the electron configuration of nitrogen? | Socratic. Funded by The full electron configuration for nitrogen is “1s”^ 2"2s"^2"2p"^3. The noble gas shorthand electron configuration is [“He”]“2s”^2"2p"^3"., Solved Four possible electron configurations for a nitrogen , Solved Four possible electron configurations for a nitrogen
Solved The electronic configuration of nitrogen (Z 7) is | Chegg.com

*Electronic configuration of a nitrogen atom. | Download Scientific *
Solved The electronic configuration of nitrogen (Z 7) is | Chegg.com. The Rise of Operational Excellence electron configuration for nitrogen and related matters.. Fitting to The electronic configuration of nitrogen (Z 7) is 1s22s22p3. There is an empirical rule (ie Hund’s rule) that requires the electrons in unfilled shells to have , Electronic configuration of a nitrogen atom. | Download Scientific , Electronic configuration of a nitrogen atom. | Download Scientific
Apply Write out the electron configuration for each atom. Then

What is the atomic orbital diagram for nitrogen? | Homework.Study.com
Apply Write out the electron configuration for each atom. Then. The electron configuration for Nitrogen is 1 s 2 2 s 2 2 p 3 . Nitrogen needs to gain 3 electrons to attain a noble-gas configuration. This will form a Nitride , What is the atomic orbital diagram for nitrogen? | Homework.Study.com, What is the atomic orbital diagram for nitrogen? | Homework.Study.com, 1.3: Atomic Structure - Electron Configurations - Chemistry LibreTexts, 1.3: Atomic Structure - Electron Configurations - Chemistry LibreTexts, Encouraged by 1s^2 2s^2 2p6 Nitrogen has an initial electron configuration of 1s^2 2s^2 2p^3 If Nitrogen gains three electrons the 2p orbitals will have 6